Clinical Study
Six-month pivotal trial data showed the Neuspera (iSNM) device delivers efficacy comparable to established SNM therapies. 1,2 The Phase II pivotal clinical study of 128 patients implanted with Neuspera’s iSNM therapy found:3
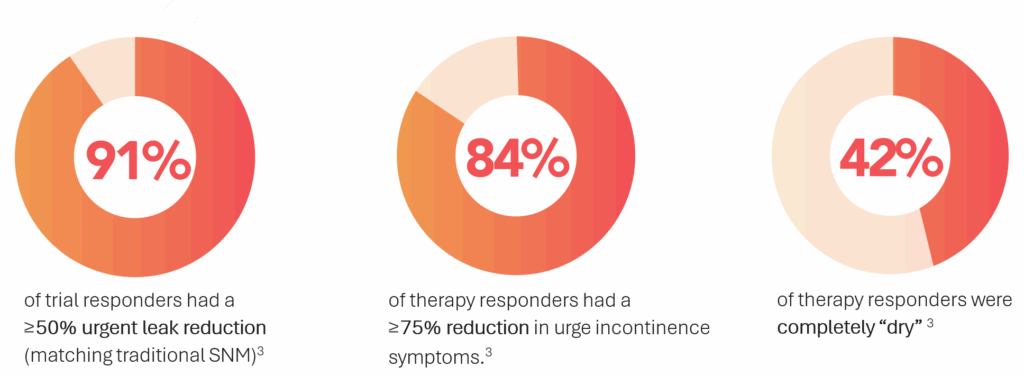
- 91% of patients who passed the initial evaluation phase had a 50% or greater reduction in urgent leaks3 – on par with reported rates in recent SNM studies1,2
- 84% of patients who responded to treatment were classified as “super responders,” meaning they experienced more than a 75% reduction in UUI symptoms
- 42% of patients who responded were completely “dry,” with 100% reduction in UUI symptoms
- 3.5x clinically significant improvement in quality of life with a reduction in voids and urgent episodes
References
- Axonics Artisan Study: McCrery, Rebecca, et al. “Treatment of Urinary Urgency Incontinence Using a Rechargeable SNM System: 6-Month Results of the ARTISAN-SNM Study.” The Journal of Urology, vol. 203, no. 1, 2020, pp. 185-192.
- Medtronic Interstim Micro Study: Goudelocke, Colin, et al. “Evaluation of Clinical Performance and Safety for the Rechargeable InterStim Micro Device in Overactive Bladder Subjects: 6-Month Results from the Global Postmarket ELITE Study.” Neurourology and Urodynamics, vol. 42, no. 4, 2023, pp. 761-769
- Neuspera SANS-UUI Study: Padron, Osvaldo et al. “Treatment of OAB Symptoms Using Neuspera’s Ultra-Miniaturized System: 6-Month Results of the SANS-UUI Phase II Study. Presented at: Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) 2025 Winter Meeting; February 25–March 1, 2025; Palm Springs, CA. Abstract, SUFU 2025.
