Alex Yeh Neurotech Pub PodcastCheck out this “stimulating” deep-dive conversation into how our respective technologies are shifting the paradigm for how patients with underlying neurological conditions may receive care in the not-too-distant future.
LinkedIn
X
Neuspera to Participate in the 2024 MedTech Innovator Cohort65 Startups Named to MedTech Innovator 2024 Cohort
Neuspera’s Statement Regarding New AUA/SUFU Guideline for OABThe American Urological Association (AUA) and Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) recently published their 2024 Guideline on the Diagnosis and Treatment of Idiopathic Overactive Bladder (OAB). The guideline promotes a shared decision-making process between the patient and physician that weighs the patient’s comorbidities along with expressed values, preferences, and treatment goals in order to help them make an informed decision regarding treatment. This completely revised guideline recognizes the difficulties and drawbacks with previous tiered therapies including behavioral interventions and medications, noting that patients may have been worn-out before having the opportunity to try more potentially attractive and effective alternatives. Options including intradetrusor botulinum toxin (botox) injection, sacral neuromodulation (SNM), and percutaneous tibial neuromodulation (PTNM), previously labeled as “third line” therapies, are now classified as “minimally invasive” therapies.
GUIDELINE STATEMENT 24
Clinicians may offer patients with OAB, in the context of shared decision making, minimally invasive therapies without requiring trials of behavioral, non-invasive, or pharmacologic management. (Expert Opinion)
The new guideline can be found here: https://www.auanet.org/guidelines-and-quality/guidelines/idiopathic-overactive-bladder#x21368.
Neuspera Medical is encouraged by the new guideline, which will facilitate OAB patient access to their most desired and appropriate option through shared decision making with their caregiver. SNM is recognized as the “gold standard” neuromodulation therapy for OAB. The Neuspera Sacral Neuromodulation system is designed to be minimally invasive, ultra-miniaturized and to eliminate the need for an implanted battery, which we believe will be an important option for patients in their shared decision-making process.
Note: The Neuspera Sacral Neuromodulation System is currently limited to Investigational Use Only.
Steven W. Siegel, MD
Neuspera Medical CMO
Neuspera Medical, Inc. Completes Patient Enrollment and Implants for the SANS-UUI Pivotal Clinical TrialThe international study is evaluating the safety and efficacy of the Neuspera System for the treatment of urinary urge incontinence (UUI), a symptom of overactive bladder (OAB)
SAN JOSE, Calif., Dec. 6, 2023 /PRNewswire/ — Neuspera Medical, Inc., a neuromodulation company pioneering the Neuspera Implantable Sacral Neuromodulation (SNM) System, today announced it has completed patient enrollment and implant procedures for the pivotal clinical trial (SANS-UUI) of its Neuspera System, a discreet, minimally invasive implant designed to provide patients personal control and relief from urinary urge incontinence (UUI), a symptom of overactive bladder (OAB).
“We are pleased to have reached the target patient enrollment in our pivotal SANS-UUI trial,” said Steffen Hovard, CEO of Neuspera Medical. “The completion of patient implants builds on the progress and strong momentum from our previously reported positive clinical outcomes.”
The SANS-UUI multi-center, single-arm clinical study is designed to demonstrate the safety and efficacy of the Neuspera System and gain U.S. Food & Drug Administration (FDA) approval in the U.S. Patients were screened and implanted with the device at 26 centers across the U.S. and Europe.
“Completing patient enrollment and implants is an important milestone in Neuspera’s journey towards revolutionizing the way physicians utilize SNM therapy,” commented Dr. Steve Siegel, Chief Medical Officer of Neuspera Medical. “We’re grateful to the patients and physicians partnering with us in this study, and we look forward to finalizing the data that will support our FDA submission of the Neuspera System.”
OAB is a common medical disorder affecting roughly 1 in 6 adults. OAB symptoms include a sudden, uncontrolled need or urge to urinate, urine leakage, and/or the need to pass urine many times during the night and day. These symptoms can make it difficult to work, socialize, exercise and lead a normal, active life which can lead to depression, self-esteem and social stigma issues.
Neuspera’s unique system is designed to provide patients who have failed medication management a minimally invasive, more approachable way of benefiting from sacral neuromodulation, a clinically proven effective treatment that’s been used for more than 30 years to treat UUI associated with OAB.
The Neuspera System is the least invasive sacral neuromodulation device and includes an ultra-miniaturized pulse generator attached to an electrode array. It is designed to be discreet, to provide patients with freedom from inconvenient and visible batteries, and to empower them to regain control of their life.
“As the coordinating investigator for the SANS-UUI clinical trial, I’m excited at the prospects of the Neuspera System for treating patients with OAB symptoms in the least invasive manner,” said Dr. Osvaldo Padron with Florida Urology Partners.
The Neuspera System has FDA clearance for treating chronic pain of peripheral nerve origin and is undergoing clinical trials as an investigational device for the use of treating UUI, a symptom associated with OAB.
About Neuspera Medical
Neuspera Medical, Inc. is committed to developing implantable medical devices that will improve the lives of patients battling chronic illnesses. The Neuspera platform is designed to provide patients and physicians with new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives aim to help patients restore their health and well-being for a better quality of life. For more information, please visit www.neuspera.com.
Media Contact:
Hyedi Nelson
Bellmont Partners
651-757-7054
hyedi@bellmontpartners.com
SOURCE Neuspera Medical, Inc.
Neuspera Medical, Inc. Announces Milestone Completion of 100th Implant in Its SANS-UUI Phase II Clinical TrialThe nationwide study is evaluating the safety and efficacy of the Neuspera System for the treatment of urinary urge incontinence (UUI), a symptom of overactive bladder (OAB)
SAN JOSE, Calif. , Nov. 14, 2023 /PRNewswire/ — Neuspera Medical, Inc., a neuromodulation company pioneering the Neuspera Implantable Sacral Neuromodulation (SNM) System, today announced the completion of the 100th implant of its Neuspera System as part of the SANS-UUI Phase II clinical trial. SANS-UUI is an international study that’s evaluating the safety and efficacy of the Neuspera System, a discreet, minimally invasive implant designed to provide patients personal control and relief from urinary urge incontinence (UUI), a symptom of overactive bladder (OAB).
“I’m always looking for the most advanced and effective treatment methods to offer my patients suffering from overactive bladder that will provide them relief and help them get back to living their lives as normally as possible,” said Dr. Derrick Sanderson, D.O. Urogynecology at Women’s Health Advantage in Fort Wayne, IN. “I’m excited to be a part of this clinical trial and the potential of this minimally invasive and discreet sacral neuromodulation therapy option.”
OAB is a common medical disorder affecting roughly 1 in 6 adults. OAB symptoms include a sudden, uncontrolled need or urge to urinate, urine leakage, and/or the need to pass urine many times during the night and day. These symptoms can make it difficult to work, socialize, exercise and lead a normal, active life which can lead to depression, self-esteem and social stigma issues.
Neuspera’s unique system is designed to provide patients who have failed medication management a minimally invasive, more approachable way of benefitting from sacral neuromodulation, a clinically proven effective treatment that’s been used for more than 30 years to treat UUI associated with OAB.
The system is comprised of a minimally invasive, ultra-miniaturized pulse generator that is 100 times smaller than the smallest commercially available sacral neuromodulation pulse generator. It is designed to be discreet, to provide patients with freedom from inconvenient and visible batteries, and to empower them to regain control of their quality of life. The device works together with a convenient external wearable to activate the system that is designed to fit into patients’ lifestyles and give them the personal control and relief from their symptoms that have been controlling them and their life for some time.
“We’re grateful to all of the physicians and patients for their enthusiastic participation in this study,” said Steffen Hovard, CEO of Neuspera Medical. “We have positive feasibility data from phase one of SANS-UUI, and this phase two pivotal study is an important step in getting closer to being able to potentially fill a gap in the sacral and tibial neuromodulation market that meets patient demand for a discreet, effective solution.”
The Neuspera System has FDA approval for peripheral nerve stimulation (PNS) and is undergoing clinical trials as an investigational device for the use of treating UUI, a symptom associated with OAB.
About Neuspera Medical
Neuspera Medical, Inc. is committed to developing implantable medical devices that will improve the lives of patients battling chronic illnesses. The Neuspera platform is designed to provide patients and physicians with new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives aim to help patients restore their health and well-being for a better quality of life. For more information, please visit www.neuspera.com.
Media Contact:
Hyedi Nelson
Bellmont Partners
651-757-7054
hyedi@bellmontpartners.com
SOURCE Neuspera Medical, Inc.
Neuspera Medical® Announces FDA Clearance of its System for Peripheral Nerve StimulationFirst-of-its-kind, leadless micro-implant for chronic peripheral nerve pain management
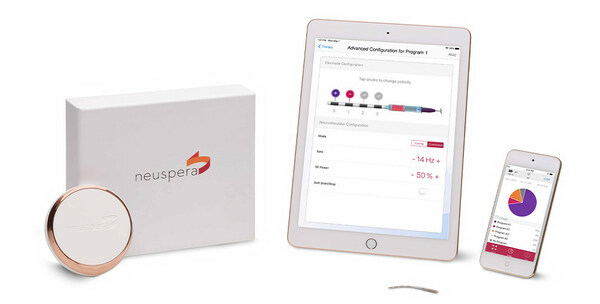
SAN JOSE, Calif., April 18, 2023 /PRNewswire/ — Neuspera® Medical, a medical device company developing implantable devices for patients battling chronic illnesses, today announced it received U.S. Food and Drug Administration (FDA) clearance for the next-generation Neuspera ultra-miniaturized system. The system is comprised of a micro-implant that delivers neurostimulation therapy through a wireless platform including a wearable transmitter and iPad-based clinician programmer.
The Neuspera system delivers peripheral nerve stimulation (PNS) through a wireless, less invasive, and more versatile platform than commercially available technology. It is the first PNS device that offers an ultra-miniaturized option, which may allow for a better patient experience and greater procedural flexibility. It is 75x smaller than the smallest commercial implantable pulse generator and provides physicians the opportunity for deeper anatomical targets compared to current technologies available today.
“We look forward to bringing this innovative technology to physicians and patients in the U.S.,” commented Steffen Hovard, CEO of Neuspera Medical. “The Neuspera ultra-miniaturized system has the potential to revolutionize the way physicians treat patients battling chronic pain while restoring patients’ health and quality of life.”
In recent years, the growth of PNS technology and treatment has been significant, with advances in implantable device technology and an increasing number of clinical studies focused on developing new PNS therapies. PNS is becoming an increasingly important treatment option for managing chronic pain conditions, with the market expecting to experience significant growth due to factors including the increasing prevalence of chronic pain, advances in implantable device technology, and rising demand for alternative pain management therapies.
About Neuspera Medical
Neuspera Medical, Inc. is committed to developing implantable medical devices that will improve the lives of patients battling chronic illnesses. The Neuspera platform is designed to provide patients and physicians with new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives aim to help patients restore their health and well-being for a better quality of life. For more information, please visit www.neuspera.com
Investor Relations and Media Contacts
Josh Cohen
Vice President, Market Development & Commercialization
Josh.Cohen@neuspera.com
SOURCE Neuspera Medical Inc.
Neuspera Hires Courtney Lane to Lead Clinical and Regulatory AffairsSAN JOSE, CA – February 1, 2023 — Neuspera® Medical, a medical device company developing miniaturized neuromodulation technology for patients with chronic illnesses, announces the hiring of Courtney Lane as Vice President, Clinical and Regulatory Affairs. The fast-growing early-stage company is progressing through enrollment of the second stage of its pivotal study (SANS-UUI) to evaluate the safety and efficacy of Neuspera’s technology in patients with urinary urgency incontinence associated with overactive bladder.
Dr. Courtney Lane has over 20 years of experience in neuromodulation device development and clinical trials. For the last 10 years, Dr. Lane worked as an independent consultant, working with cutting-edge medical device companies in the development of their devices and clinical and regulatory programs. Dr. Lane also served as Vice President of Clinical Affairs for Axonics Modulation Technologies, where she was responsible for the design and execution of the clinical program of a sacral neuromodulation device. Courtney was instrumental in the development of the Deep Brain Stimulation program at Boston Scientific, where she worked on all aspects of device development, from R&D and test designs to clinical trial designs and regulatory submissions. She then served as the Director of Clinical Science and Strategy, overseeing the development of Boston Scientific Neuromodulation’s clinical trial portfolio.
Steffen Hovard, CEO of Neuspera said: “We are excited to have Courtney Lane join Neuspera. Courtney brings a unique combination of skills and experiences that will help propel Neuspera forward – not to mention that she has a personality that makes it a pleasure to work with her.”
Dr. Lane earned her B.S.E.E. from Rice University, cum laude, and obtained her Ph.D. at the Massachusetts Institute of Technology in the Division of Health Sciences and Technology; she also worked as a researcher at Rice University, focusing on the development of hearing aid algorithms.
About Neuspera Medical
Neuspera Medical, Inc. is committed to developing implantable medical devices that will improve the lives of patients battling chronic illnesses. The Neuspera platform, which will include the Nuvella system, will provide patients and physicians new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives aim to help patients restore their health and well-being for a better quality of life.
Investor Relations and Media Contacts
Josh Cohen
Vice President, Market Development & Commercialization
Josh.Cohen@neuspera.com
Neuspera Medical® announces first successful implant of the Nuvella™ System in the second phase of its sans-UUI IDE Clinical TrialStudy to support U.S. FDA approval of sacral neuromodulation device in patients with urinary urgency incontinence symptoms
SAN JOSE, Calif., Oct. 10, 2022 /PRNewswire/ — Neuspera® Medical, a medical device company developing implantable devices for patients battling chronic illnesses, today announced the first patient successfully implanted with the Nuvella™ system in its pivotal clinical trial (SANS-UUI). The procedure was performed by Dr. Jodi Michaels of Minnesota Urology, St. Paul, MN. The study will evaluate the safety and efficacy of the Nuvella system, designed to treat overactive bladder (OAB) with sacral neuromodulation (SNM) in patients with urinary urgency incontinence (UUI) symptoms.

Photo (left to right): Alex Yeh, Neuspera Founder & CTO, Dr. Jodi Michaels of Minnesota Urology and Steven Siegel, Neuspera CMO
“I am excited to be a part of the initial cases and for the potential of this innovative technology to treat my OAB patients in a less invasive and more adaptable way than current devices,” stated Dr. Jodi Michaels. “This system has the ability to treat UUI symptoms with the smallest available implant, significantly improving the patient experience.”
The prospective, multi-center, single-arm study will enroll 145 patients globally. The feasibility phase results of SANS-UUI were first presented at the annual meeting of the American Urological Association (AUA) in September 2021 and again at the winter meeting of the Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) in 2022. In 34 patients implanted with the Nuvella system, 90% of subjects demonstrated a 50% improvement in UUI symptoms at 6 and 12 months with two hours of daily stimulation, while 52% were completely dry at the 12-month visit.
Sacral neuromodulation is a proven therapy for the treatment of UUI that can significantly improve quality of life in patients suffering from this condition. The Nuvella system delivers neuromodulation therapy through a wireless, less invasive, and more versatile platform than commercially available technology. Nuvella is the first sacral neuromodulation device that offers this ultra-miniaturized option, which may allow for a better patient experience and greater procedural flexibility.
“We are pleased to enroll the first patients in our pivotal SANS-UUI trial, an important milestone for the company. Kicking off enrollment builds on the strong momentum from our feasibility experience,” commented Steffen Hovard, CEO of Neuspera Medical. “This technology has the potential to revolutionize the way physicians utilize SNM therapy to treat patients battling chronic conditions, while restoring patients’ health and quality of life.”
Overactive bladder (OAB) is a common medical disorder affecting roughly 1 in 6 adults.1 Symptoms of OAB include sudden, uncontrolled need or urge to urinate (most common), urine leakage, and need to pass urine many times during the night. The worldwide OAB treatment market size was 1,192.5M in 2020 and is experiencing tremendous growth due to increasing prevalence of chronic conditions.2
About Neuspera Medical
Neuspera Medical, Inc. is committed to developing implantable medical devices that will improve the lives of patients battling chronic illnesses. The Neuspera platform, which includes the Nuvella system, will provide patients and physicians new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives aim to help patients restore their health and well-being for a better quality of life. For more information, please visit
www.neuspera.comInvestor Relations and Media Contacts
Josh Cohen
Vice President, Market Development & Commercialization
Josh.Cohen@neuspera.com
SOURCE Neuspera Medical Inc.
Neuspera Medical® Appoints Steffen Hovard as CEOHovard brings a wealth of medical device senior leadership experience to the company as it works to advance sacral neuromodulation therapies
SAN JOSE, Calif., Sept. 6, 2022 /PRNewswire/ — Neuspera® Medical, a medical device company developing implantable medical device technology for patients with chronic illness, today announced the hiring of seasoned medtech executive, Steffen Hovard as Chief Executive Officer. He joins as the company recently began enrollment of the second phase of its U.S. pivotal clinical trial (SANS-UUI). The study will evaluate the safety and efficacy of Neuspera’s Nuvella™ system designed to treat patients with urinary urgency incontinence (UUI), which is a symptom of overactive bladder (OAB).
“I am delighted to have Steffen join and lead Neuspera as we look to bring our therapy platform to patients struggling with debilitating conditions such as UUI,” commented Mudit Jain, of Treo Ventures. “He is an accomplished executive with an impressive record of leading multiple functions and driving significant growth in the urology space.”
Most recently, Hovard served as the President of Coloplast Interventional Urology, with leadership responsibility for four business areas within urological devices (class I, II, and III) including 900 employees across 19 countries. Steffen also serves as a board member for several companies in the medical device industry.
“I am excited to join Neuspera at this important time as the company builds momentum and clinical evidence for the Nuvella system,” stated Steffen Hovard, CEO of Neuspera Medical. “Sacral neuromodulation (SNM) is a proven therapy for the treatment of UUI which can significantly improve quality of life in patients suffering from this condition. Nuvella is the first sacral neuromodulation device that offers an ultra-miniaturized option, which aims to provide a better patient experience and greater procedural versatility. I look forward to working with a world-class team to bring this important technology to market.”
About Neuspera Medical
Neuspera Medical, Inc. is committed to developing implantable medical devices that will improve the lives of patients battling chronic illnesses. The Neuspera platform, which will include the Nuvella system, will provide patients and physicians new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives aim to help patients restore their health and well-being for a better quality of life. To learn more visit
www.neuspera.com.
Investor Relations and Media Contacts
Josh Cohen
Vice President, Market Development & Commercialization
Josh.Cohen@neuspera.com
SOURCE Neuspera Medical Inc.
Neuspera Announces Leadership Expansion with the Addition of New CMOSan Jose, CA – August 4, 2022– Neuspera® Medical, a medical device company developing implantable medical device technology for patients battling chronic illness, today announced the hiring of Steven Siegel, MD, as Chief Medical Officer. Dr. Siegel joined Neuspera as the company recently began enrollment of the second phase of their pivotal clinical trial (SANS-UUI). The company presented initial, positive results from the feasibility phase at the American Urologic Association (AUA) 2021 annual meeting and at the winter meeting of the Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) in February of this year.
“We are excited to welcome Steven to the Neuspera team. He has an exceptional background, proven leadership ability, and deep domain expertise and will add tremendous value to the company moving forward as we build and scale for clinical and commercial operations,” said Neuspera board member Lori Hu from Vertex Ventures. “His wealth of knowledge and industry experience will be critical in guiding our strategic plan as we accelerate the pivotal trial and prepare for future milestones.”
Dr. Siegel has been practicing urology for over 35 years and is an internationally recognized expert and pioneer in the evolution of sacral neuromodulation. He participated in multi-center clinical trials with UroSystems in the late 1980s, when he was the Head of the Section of Female Urology and Urodynamics at the Cleveland Clinic. He participated in the pivotal “103” trials, which led to Interstim’s original approval for the indication of urinary urge incontinence (UUI) in 1997. He was a founding member of the International Society of Pelvic Neuromodulation, which later merged with the Society of Female Urology and Urodynamics. More recently, he was the national principal Investigator for the InSite trial, and lead author in numerous publications related to the study. He has recently retired from his clinical practice and served as the Director of the Minnesota Urology Centers for Female Urology and Continence Care since 1993.
“I am very excited to join a pioneering company like Neuspera and its endeavor to provide relief for patients battling the life-limiting symptoms of urinary urgency incontinence,” said Dr. Siegel. “I am passionate about revolutionizing neuromodulation treatment to truly transform how patients and physicians address chronic illness.”
About Neuspera Medical
Neuspera Medical, Inc. is committed to developing implantable medical devices that will improve the lives of patients battling chronic illnesses. The Neuspera platform, which will include the Nuvella system, will provide patients and physicians new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives aim to help patients restore their health and well-being for a better quality of life.
Investor Relations and Media Contacts
Josh Cohen
Vice President, Market Development & Commercialization
Josh.Cohen@neuspera.com