about
neuspera medical
Empowering Patients To
change their lives
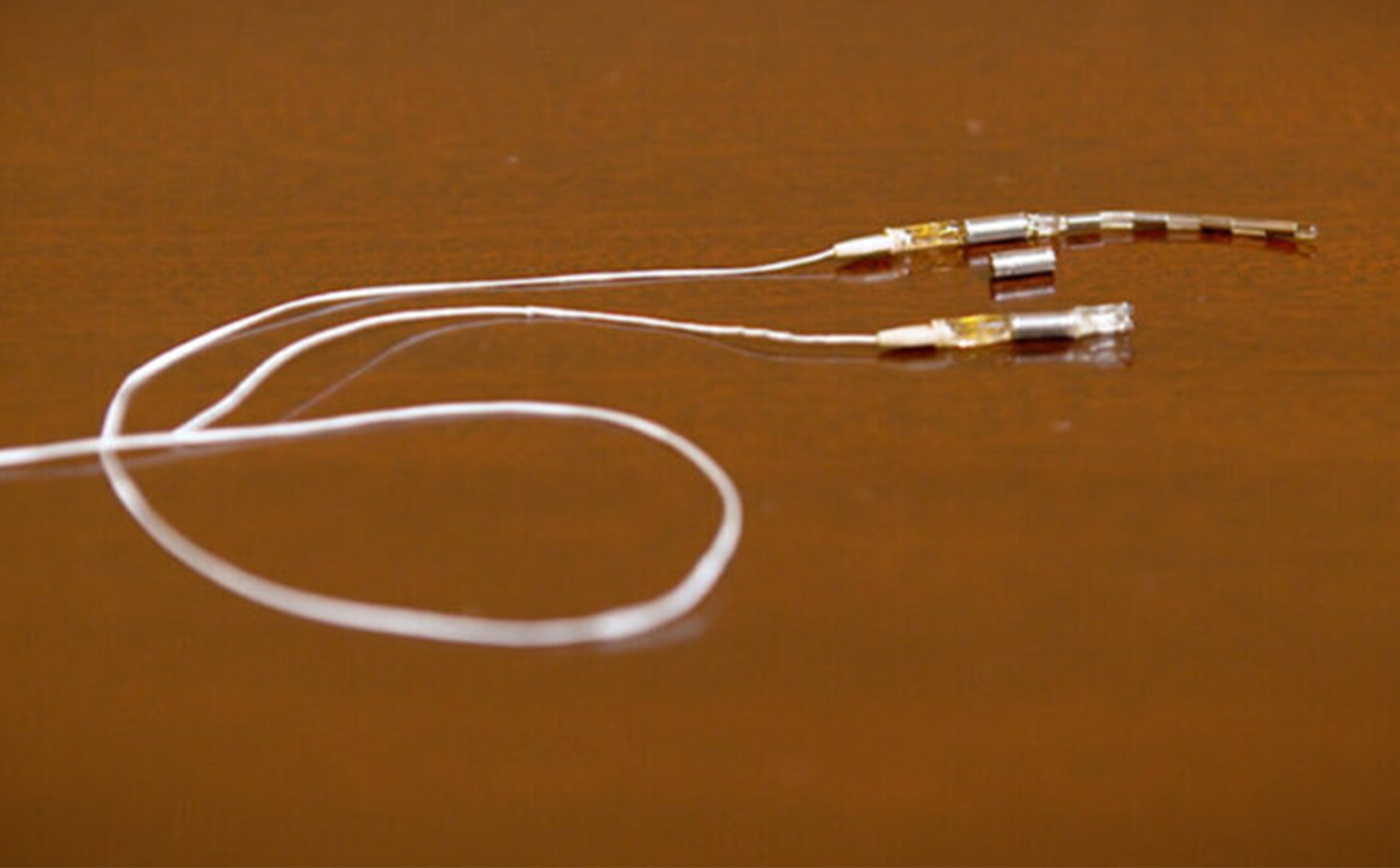
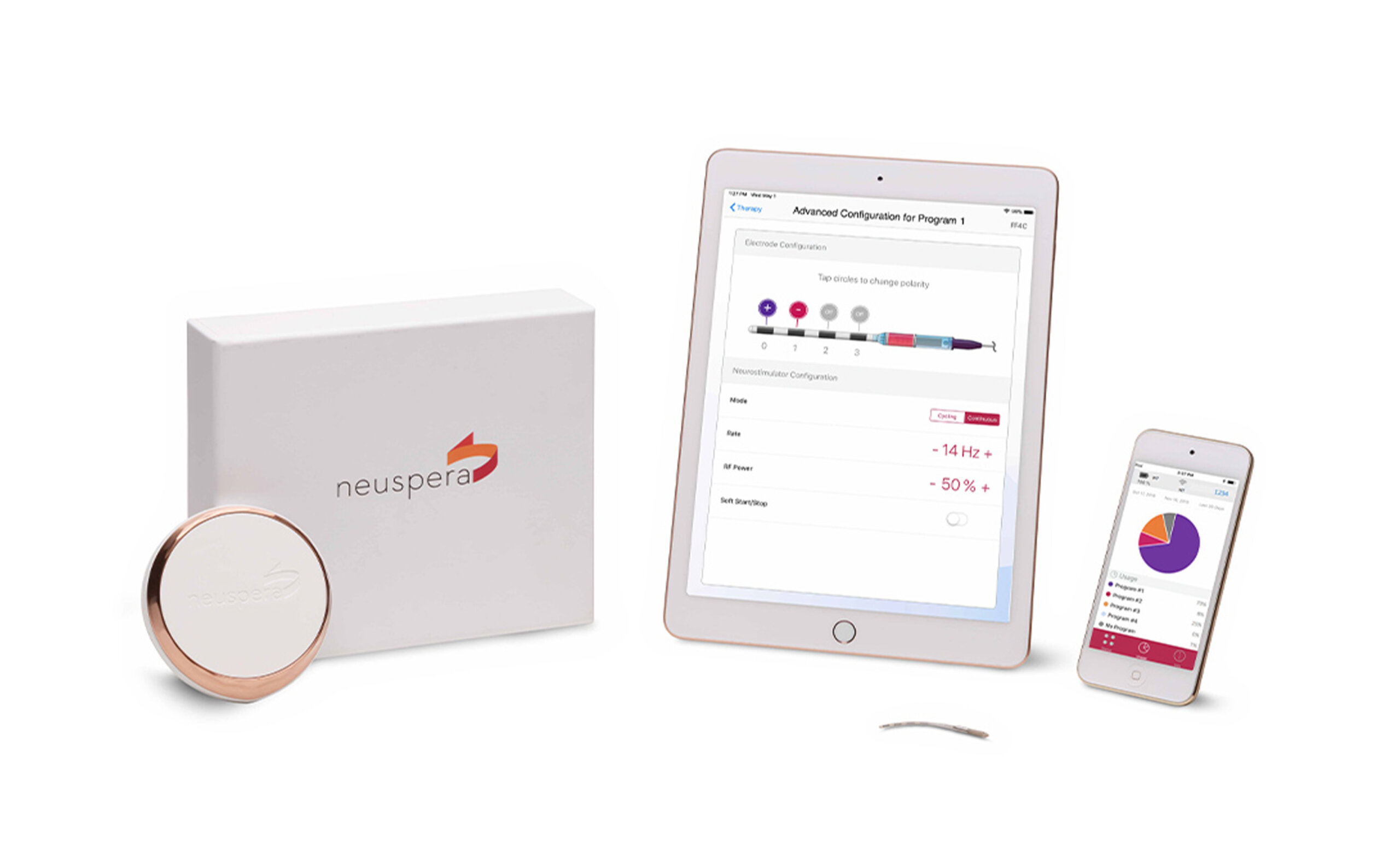
Neuspera® Medical, Inc. is a leader in advanced, directionally adaptive energy systems that power tiny medical implants deep within the body. The company is introducing a new class of neurostimulation technology, starting with the first integrated sacral neuromodulation (iSNM) system for urinary urge incontinence (UUI), approved by the FDA in June 2025.
Our
executive team
As Chief Executive Officer of Neuspera Medical since March 2025, Dave Van Meter draws on a track record of successful commercialization leadership and capital-raising experience to advance the company’s integrated sacral neuromodulation system as a next-generation treatment for urinary urge incontinence.
A 30-year med-tech leader, Dave has transformed more than a dozen novel devices from concept into widely adopted therapies. He previously served as president and CEO of ophthalmic device maker Ivantis, Inc. Starting as Ivantis’ second employee, he led the company through clinical trials, FDA approval of the Hydrus® Microstent glaucoma device, and global commercialization in 30+ countries, with over $60 MM in annual revenue and profitability into a highly competitive space with entrenched incumbents — culminating in a successful $475 MM acquisition by Alcon in 2022.
Earlier in his career, Dave held various roles of increasing responsibility as an early leader in the creation and growth of Abbott Vascular’s coronary and endovascular portfolio, including serving in roles such as Director of U.S. Marketing, General Manager of Coronary Technologies, and Vice President, Strategic Marketing, where he optimized global R&D investments and was responsible for the pipeline generation for a nearly $2 B global business.
Between Ivantis and Neuspera, Dave recently also worked in the investment side of Medtech as a Venture Partner for Vertex Ventures, and serves on the Boards of Spyglass Pharma and Moximed.
He holds an M.B.A. from the University of Notre Dame and a B.A. from Hillsdale College.
Alexander J. Yeh is a medical device innovator who founded Neuspera Medical to turn millimeter-size, wireless implants into practical, disruptive tools for patient care. As Chief Technology Officer and Vice President of Research & Development, he guides research, product design, and patent strategy for the company’s neurostimulation technology.
Alex conceived Neuspera’s core “mid field” power transfer technology while completing his Ph.D. in electrical engineering at Stanford University, where he built and validated deeply implanted devices able to receive power and communicate without wires. Under his direction that innovation progressed from preclinical models to FDA approval in 2025.
Prior to Stanford, Alex was a researcher in communication algorithms for ultra high bandwidth wireless systems and worked as a system engineer in the startup, TagArray, developing UWB RFID products.
He holds M.S. and Ph.D. degrees in electrical engineering from Stanford University and dual B.S./B.A. degrees in electrical engineering and economics from the University of California, Los Angeles.
Named in more than 175 patents, patent applications, and academic papers related to implantable medical devices, Alex also serves on the board of Greenliant Systems, a leading global supplier of solid-state storage solutions for industrial, automotive, and medical applications.
Steve Paul serves as Chief Commercial Officer of Neuspera Medical, where he leads the sales, marketing, reimbursement, and operations strategy to support the launch and expansion of the company’s FDA-approved, implantable device for urinary urge incontinence. He is focused on capturing a largely underpenetrated market and positioning Neuspera’s iSNM system as a preferred treatment option.
A seasoned commercial strategist, Steve has consistently led med-tech businesses from early revenue to category leadership, blending market-access expertise, targeted sales execution, and data-driven marketing to accelerate adoption of novel therapies.
Before joining Neuspera, Steve guided Vertos Medical through a period of exceptional growth, scaling revenue from $10 MM to ~$80 MM in five years. He secured national reimbursement milestones, advanced commercialization, and helped position the company for acquisition by Stryker, a global med-tech leader.
Steve holds a B.A. in political science from the University of California, Santa Barbara.
Dr. Steven Siegel is an internationally recognized expert and pioneer in the evolution of Sacral Neuromodulation, having participated in multi-center clinical trials with Urosystems in the late 1980’s when he was the Head of the Section of Female Urology and Urodynamics at the Cleveland Clinic. After the therapy was acquired by Medtronic in the early 1990’s, he participated in the pivotal “103” trials and publications, and helped present the data from those studies to the FDA, leading to Interstim’s original approval for the indication of Urinary Urge Incontinence in 1997. He was instrumental in broadening acceptance and clinical application of the therapy, teaching hundreds of physicians the hows and whys of Sacral Neuromodulation all over the world. He was a founding member of the International Society of Pelvic Neuromodulation, which later merged with the Society of Female Urology and Urodynamics, and played a major role in the merged group’s programming related to the therapy for over a decade. More recently, he was the National Principle Investigator for the InSite Trial, and lead author in many of the publications related to that study. He has recently retired from his clinical practice where he specialized in FPMRS, and served as the Director of the Minnesota Urology Centers for Female Urology and Continence Care since 1993. He has trained over 30 fellows associated with that program. Dr. Siegel also served as the business section editor for the Urology Practice journal from its inception until 2020. He has been the recipient of a number of awards including: AUGS’ National Association for Continence “Continence Care Champion” award in 2018, the Christina Manthos Award for extraordinary mentoring of female urologists from the Society of Women in Urology in 2011, and the David C. Utz Award for Urological Advancement and Innovation from the Minnesota Urological Society in 2002. Dr. Siegel is a Past President of the North Central Section of the American Urological Association (2010), and former board member and Chairman of the Practice Standards Committee of the SUFU. Dr. Siegel graduated from the University of Michigan Medical School and completed his urology residency at the Cleveland Clinic in 1986.
Bunty Banerjee is a seasoned life sciences executive with more than 25 years of experience in the medical device industry, holding senior operational leadership positions at Omnicell, Philips Image Guided Therapy, Abbott, and Boston Scientific, which involved the development, commercialization, and scaling of complex medical products. He has served in senior leadership roles in enterprises ranging from early start-ups to Fortune 100 corporations, overseeing operations from pre-clinical to billion-dollar businesses.
Mr. Banerjee has a Bachelor of Science degree in Electrical Engineering from Jadavpur University, India, and a Master of Science degree in Industrial Management from Clemson University.
With nearly two decades of experience in accounting, finance, and customer and sales operation integrations, Kelli Gerasimou demonstrates a passion for accelerating the growth of emerging companies. She is a certified CPA who works as a cross-functional business partner, creating scalable strategies to support commercial expansion of small startups. For five years, she was the Vice President of Finance at Ivantis Inc before facilitating its $545M sale to Alcon, where she continued serving as the VP of Finance. Kelli has been successful in integrating strategies that align global financial operations with a corporate vision. She has expertise in international commercial launches through her management of foreign entities including legal and tax structure, VAT/ GST compliance, banking, and operational processes. She was nominated as Orange County Business Journal’s CFO of the year “Rising Star” three years in a row and nominated as one of 2022 OCBJ’s Women in Business. Kelli graduated with her B.S. in accounting from CalPoly Pomona.
Dr. Courtney Lane has over 20 years of experience in neuromodulation device development and clinical trials. For the last 10 years, Dr. Lane worked as an independent consultant, working with cutting edge medical device companies in the development of their devices and clinical and regulatory programs. Dr. Lane also served as Vice-President of Clinical Affairs for Axonics Modulation Technologies, where she was responsible for the design and execution of the clinical program of a sacral neuromodulation device. Courtney was instrumental in the development of the Deep Brain Stimulation program at Boston Scientific, where she worked on all aspects of device development, from R&D and test designs to clinical trial designs and regulatory submissions. She then served as the Director of Clinical Science and Strategy, overseeing the development of Boston Scientific Neuromodulation’s clinical trial portfolio.
Memberships
that matter
Neuspera Medical is a member of AdvaMed. Our employees, contracted healthcare professionals, and other consultants and vendors are expected to follow laws and regulations applicable to the medical device industry. In addition, internal policies and procedures govern our commitment to lead by example in all facets of our business.
Our
investors







Featured
news and milestones


INTERESTED IN
joining our team?
Neuspera Medical is a dynamic, fast-paced and innovative company. We welcome individuals with a passion for improving patients’ lives to reach out to careers@neuspera.com and let us know their interests.

