about
neuspera medical
Empowering Patients To
change their lives
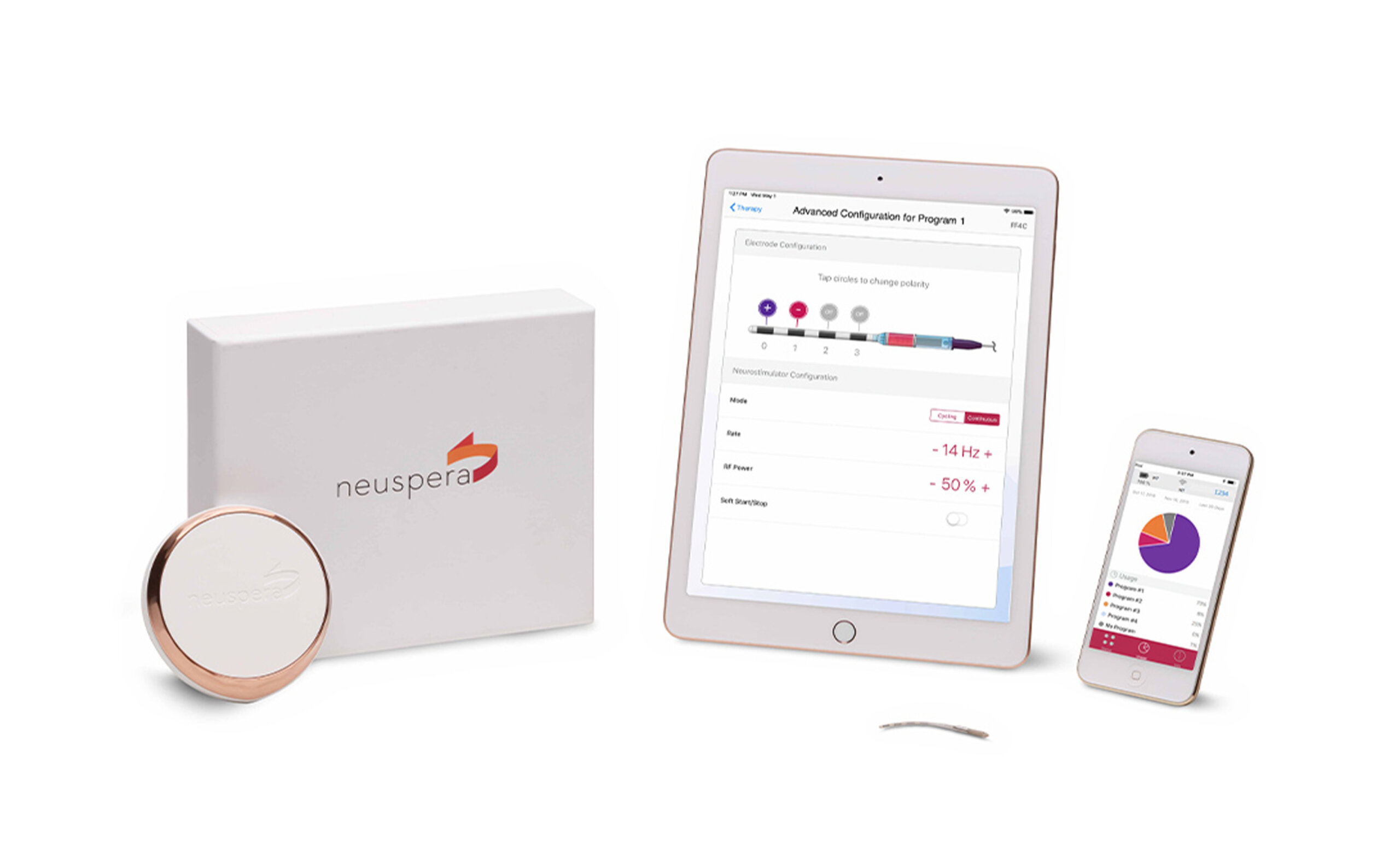
Neuspera® Medical, Inc. is committed to developing implantable medical device technology that will improve the lives of patients battling chronic illness. The company’s proprietary Mid-Field Powering Technology allows devices to be significantly smaller, implanted deeper, and more reliable than other miniaturized neuromodulation technologies. The Neuspera platform will provide patients and physicians new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives may help patients restore their health and well-being for a better quality of life.
Our
executive team
Steffen Hovard is a seasoned medical device executive with 15 years of experience in urology and uro-gyncology. He spent 20 years with Coloplast with various leadership assignments across both commercial and R&D functions, and for 8 years he led President of Coloplast Interventional Urology. This included leadership responsibility for four business areas within urological devices (class I, II, and III) including 900 employees across 19 countries and $300M revenue. Steffen serves on the board of several medical devices companies in both the US and internationally – with a focus on venture and private equity backed companies. Apart from his operational roles, Steffen served on the Boards of both Medical Alley Association and AdvaMed for several years, and he remains an active industry advocate.
He holds Bachelor and Master Degrees in marketing & strategy from Copenhagen Business School in Denmark.
Alexander J. Yeh is Founder, CTO, and Director of Neuspera® Medical Inc. His areas of specialty include wireless power transfer, neurophysiology, RFIC design, wireless communications, and semiconductor packaging.
Alex founded Neuspera to translate the work he advanced during his Ph.D. candidacy at Stanford University. He successfully developed devices and conducted experiments that demonstrated midfield power transfer to deeply implanted miniature devices in preclinical models as outlined in his dissertation, “Wireless Powering and Communications for mm-Sized Pacemakers, Sensors, Neuromodulators, And Optogenetics.” At Neuspera, this fundamental technology has been adapted to human use under his leadership.
Prior to joining Stanford, he was a researcher in communication algorithms for 60 GHz wireless systems and worked as an engineer in a startup, TagArray, developing UWB RFID products.
Alex has a M.S. and Ph.D. from Stanford University in Electrical Engineering, and has a BS in Electrical Engineering and BA in Economics from University of California – Los Angeles. He is named in over 100 patents, patent applications, and academic papers related to implantable medical devices. He also serves on the board of Greenliant Systems, Ltd, a leading global supplier of solid-state storage solutions for industrial, automotive, and medical applications.
Dr. Steven Siegel is an internationally recognized expert and pioneer in the evolution of Sacral Neuromodulation, having participated in multi-center clinical trials with Urosystems in the late 1980’s when he was the Head of the Section of Female Urology and Urodynamics at the Cleveland Clinic. After the therapy was acquired by Medtronic in the early 1990’s, he participated in the pivotal “103” trials and publications, and helped present the data from those studies to the FDA, leading to Interstim’s original approval for the indication of Urinary Urge Incontinence in 1997. He was instrumental in broadening acceptance and clinical application of the therapy, teaching hundreds of physicians the hows and whys of Sacral Neuromodulation all over the world. He was a founding member of the International Society of Pelvic Neuromodulation, which later merged with the Society of Female Urology and Urodynamics, and played a major role in the merged group’s programming related to the therapy for over a decade. More recently, he was the National Principle Investigator for the InSite Trial, and lead author in many of the publications related to that study. He has recently retired from his clinical practice where he specialized in FPMRS, and served as the Director of the Minnesota Urology Centers for Female Urology and Continence Care since 1993. He has trained over 30 fellows associated with that program. Dr. Siegel also served as the business section editor for the Urology Practice journal from its inception until 2020. He has been the recipient of a number of awards including: AUGS’ National Association for Continence “Continence Care Champion” award in 2018, the Christina Manthos Award for extraordinary mentoring of female urologists from the Society of Women in Urology in 2011, and the David C. Utz Award for Urological Advancement and Innovation from the Minnesota Urological Society in 2002. Dr. Siegel is a Past President of the North Central Section of the American Urological Association (2010), and former board member and Chairman of the Practice Standards Committee of the SUFU. Dr. Siegel graduated from the University of Michigan Medical School and completed his urology residency at the Cleveland Clinic in 1986.
With nearly two decades of experience in accounting, finance, and customer and sales operation integrations, Kelli demonstrates a passion for accelerating the growth of emerging companies. She is a certified CPA who works as a cross-functional business partner, creating scalable strategies to support commercial expansion of small startups. For five years, she was the Vice President of Finance at Ivantis Inc before facilitating its $545M sale to Alcon, where she continued serving as the VP of Finance. Kelli has been successful in integrating strategies that align global financial operations with a corporate vision. She has expertise in international commercial launches through her management of foreign entities including legal and tax structure, VAT/ GST compliance, banking, and operational processes. She was nominated as Orange County Business Journal’s CFO of the year “Rising Star” three years in a row and nominated as one of 2022 OCBJ’s Women in Business. Kelli graduated with her B.S. in accounting from CalPoly Pomona.
Steve Schellenberg has been in the active implantable industry since 1995. He has led operations at multiple early stage companies transitioning through to commercialization and acquisition. He was Director of Operations at Epicor Medical, and Director of Manufacturing Engineering at Spinal Modulation, both acquired by St. Jude Medical (now Abbott). Steve was also VP of Operations at Intrapace Inc. where he helped lead the development and building of operations in support of an implantable neurostimulator intended for the treatment of obesity, resulting in CE mark approval and successful OUS Clinical Trial.
Steve is an expert in electronics assembly and test, design for manufacturing, and hermetic welding. He is well versed in packaging, sterilization, and quality systems. He has built the infrastructure to support product development, regulatory approvals, clinical trials, and commercialization for multiple enterprises. Steve has a BS in Mechanical Engineering from California State University at Chico.
Dr. Courtney Lane has over 20 years of experience in neuromodulation device development and clinical trials. For the last 10 years, Dr. Lane worked as an independent consultant, working with cutting edge medical device companies in the development of their devices and clinical and regulatory programs. Dr. Lane also served as Vice-President of Clinical Affairs for Axonics Modulation Technologies, where she was responsible for the design and execution of the clinical program of a sacral neuromodulation device. Courtney was instrumental in the development of the Deep Brain Stimulation program at Boston Scientific, where she worked on all aspects of device development, from R&D and test designs to clinical trial designs and regulatory submissions. She then served as the Director of Clinical Science and Strategy, overseeing the development of Boston Scientific Neuromodulation’s clinical trial portfolio.
Our
medical advisory board
Memberships
that matter
Neuspera Medical is a member of AdvaMed and the Medical Device Manufacturing Association (MDMA). Our employees, contracted healthcare professionals, and other consultants and vendors are expected to follow laws and regulations applicable to the medical device industry. In addition, internal policies and procedures govern our commitment to lead by example in all facets of our business.
Our
investors






And a Strategic Partner
Featured
news and milestones


INTERESTED IN
joining our team?
Neuspera Medical is a dynamic, fast-paced and innovative company. We welcome individuals with a passion for improving patients’ lives to reach out to careers@neuspera.com and let us know their interests.


